Research Projects
Our research aim is to understand various life phenomena at an atomic resolution. We determine tertiary structures of proteins and nucleic acids that are crucial for biological processes by X-ray crystallography, generate hypotheses how their functions emerge from the structures, and demonstrate the hypotheses by in vitro and in vivo analyses of mutants designed based on the structures. We are also employing computer simulation to understand the dynamic process of the chemical reactions or carry out drug design.
We now mainly focus on the following projects.
Processing, chemical modification and aminoacylation of transfer RNA
Transfer RNA (tRNA) acts as an adaptor molecule to link the genetic code (in messenger RNA) to a specific amino acid. tRNA is initially transcribed by RNA polymerase as a precursor RNA with long extensions at the 5' and 3' terminus. Maturation of tRNA into a functional RNA requires processing of the extensional sequences, chemical modifications and specific aminoacylation. The post-transcriptional chemical modifications contribute to the structural stabilization and the specific codon recognition by tRNA. We are promoting structure determination of the tRNA-maturating enzymes in a complex with tRNA (precursor) to especially elucidate the dynamic mechanism of their highly specific chemical reactions.
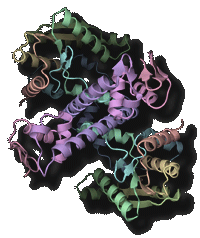
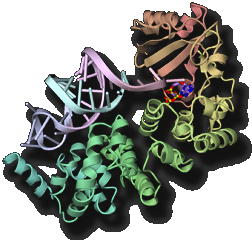
- "Structural basis for anticodon recognition by methionyl-tRNA synthetase"Nat. Struct. Mol. Biol., 12 931-932 (2005).PubMed: link
- "Structural basis for template-independent RNA polymerization"Nature, 430 700-704 (2004).PubMed: link
Action mechanism of molecular chaperons ↑
Molecular chaperons assist refolding of denatured polypeptides to restore their functions, or reversely, unfold native proteins to transport them through lipid bilayer membrane. Such structural changes of substrate peptides (proteins) are associated with dynamic conformational transition of chaperons. Our aim is to comprehend the structural basis for the dynamic action mechanism of molecular chaperons by X-ray crystallography of the snapshots for their reaction processes.
Vesicular transport regulated by small GTPases ↑
Since eukaryotic intracellular region is compartmentalized by dynamic lipid bilayer membranes, biomolecules are transported via vesicles. At the target site, vesicles transfer the biomolecules by fusing with the target membrane. The membrane fusion between vesicles and plasma membrane involves protein supramolecular complex called tethering factor (Exocyst) that loosely and reversibly tethers both the membranes, and SNARE complex that fuses the vesicular and plasma membranes. Such sequential membrane fusion reactions mediated by protein-protein interactions are regulated by specific small GTPases such as Rab. Our laboratory determines the ternary structures of the complexes of small GTPases and their effectors, which are devoted for exocytosis, to elucidate the fundamental mechanism of membrane fusion regulated by small GTPases.
Sensing receptors (channels) and membrane transporters ↑
Five senses (touch, taste, hearing, eyesight and smell) are essential for higher eukaryotes to determine their actions in response to environmental insults. We promote structure determination of the sensing receptors complexed with ligands to elucidate the general mechanism of how external chemical and physical stimuli activate and change the conformation of the sensing receptors (channels) to form a novel interaction with the coupled G proteins or to change the cation permeability. We are further promoting X-ray crystallography of metal transporters and membrane translocon to elucidate the fundamental mechanism of the specific substance transportation through lipid bilayer membrane.
Structure-based cancer research ↑
We promote X-ray crystallography of various oncogenic products or signal transduction proteins in a complex form to provide the structural basis for the mechanism of how their dysfunction cause cancer and metastasis of cancer cells. We mainly focus on growth factor receptors, oncogenic mediators and transcriptional factors, which transduce the TGF- and Wnt/-catenine signals. We are also promoting the project on innate immunity. Our final goal is to design a novel and effective anti-cancer drugs with minimum side effects, on the basis of their atomic structures.
![[XXX]](/image/tw.png)